"After graduating with a master's degree in biology, I worked as a dentist for 20 years. In order to help those who biologist feel confident and happy, I did not continue to become a dentist and started a biotech startup."
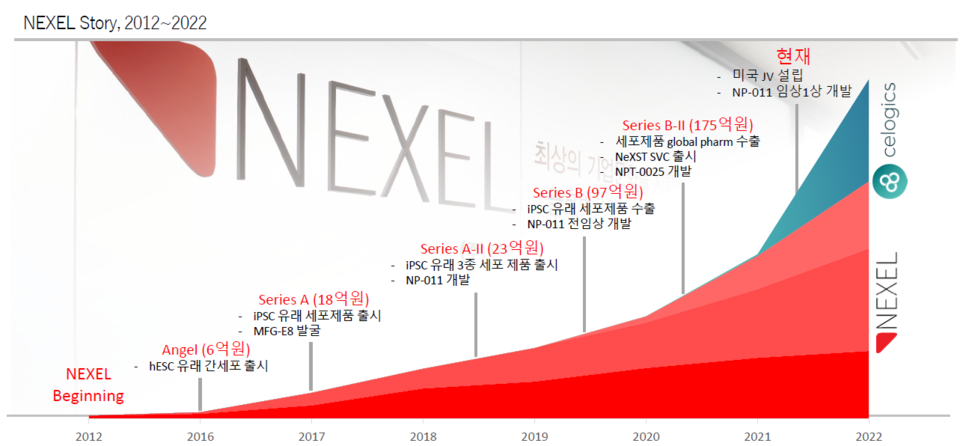
Han Chung-seong, CEO of Nexel, entered dental school after obtaining a master's degree in biology and became a dentist in 2001, but did not give up on biotechnology research. He had a desire to do research on stem cells, which he had studied in his master's course, and established Nexel in 2012. In 2014, the liver cell manufacturing technology was transferred from co-founder Kim Jong-hoon, a professor at the Department of Biotechnology at Korea University.
Nexel, a company specializing in human induced pluripotent stem cell (hiPSC)-derived somatic cells and organoids, is leading a new paradigm in the global drug toxicity evaluation market by commercializing a new drug toxicity evaluation platform using stem cell-derived functional somatic cells for the first time in Korea. Based on its outstanding stem cell-based technology, the company is exploring stem cell-derived protein and peptide new drug candidates and discovering treatments for various acute and chronic diseases.
Nexel attracted the attention of the bio industry by attracting a total of 18.5 billion won through pre-IPO last month amid difficult investment situations by biotech companies. The company aims to be listed on the KOSDAQ in 2024. Hit News met with CEO Han Chung-seong to hear about the company's future plans.

Han Choong-seong, CEO of Nexel
#1. Biotech startup specialized in iPSC field... Design a distinct business model
Founded in 2012 as a biotech specializing in induced pluripotent stem cells (iPSC). Why did you pay attention to the field of iPSCs?
 In 2012, CEO Han Choong-seong founded Nexel, a biotech specialized in induced pluripotent stem cells (iPSC).
In 2012, CEO Han Choong-seong founded Nexel, a biotech specialized in induced pluripotent stem cells (iPSC).
/ Source = Company IR data
"We paid attention because it was determined that the iPSC field would quickly replace the cell line market or adult stem cell field in the future. Aside from therapeutic development, iPSCs can be used in various fields. One example is the evaluation of new drug toxicity and safety. Usually, when developing a new drug, 2 trillion won is invested over 13 years.
We paid attention to the fact that the market is growing rapidly as global pharmaceutical companies are using stem cells for new drug screening and drug safety evaluation. In the meantime, pharmaceutical companies have invested enormous amounts of money to research new drug candidates and conducted animal toxicity tests, but human clinical trials have often shown different results. Using iPSC-derived somatic cells, it is possible to predict the success of new drug candidates at the beginning of research.
In addition, it can be an alternative market for animal testing, which is used by more than 500 million animals a year, and more than 5 million animals in Korea alone, and can solve the ethical problem of animal testing. Currently, most of the global stem cell supply is supplied by Fujifilm Cellular Dynamics, Inc. (FCDI), an American subsidiary of Fujifilm Japan, and Nexel is the only one in Korea."
Nexel obtained the iPSC commercialization license for the first time in Korea and has global-level expertise. Please explain the differentiated iPSC technology.
"iPSC-derived somatic cell culture is not an easy process. It is not easy to efficiently mass-produce it because it has to go through various differentiation processes. Differentiation of production technology is important. Currently, 700 to 800 vials can be produced per month in the laboratory at Nexel Magok Headquarters. Such mass production is possible because of the technology that utilizes Nexel's unique small molecule compound. By developing an optimized cryopreservation method in-house, it is possible to maintain the viability and functionality of each cell. In addition, strict quality control (QC) standards and the establishment of specialized badges are also differentiated factors.
Technology for iPSC-derived cardiomyocytes is important in how similar they are to primary cardiomyocytes isolated from tissues. In particular, in the case of cardiomyocytes, since they are based on complex electrophysiology ranging from electrical signals to contraction, we focus on identifying the functional parts of cells with advanced technology. Our company's cardiomyocytes have significantly similar ion channel activity to cardiomyocytes isolated from tissue, allowing more sensitive detection of drugs that affect the channel.
It showed more than 20% better reactivity to drugs known to be difficult to detect in conventional iPSC-derived cardiomyocytes compared to competitor products. In addition, the superiority of the cell was confirmed by objectively verifying it through a third party rather than internal test results. Nexel is already conducting a number of iPSC-related consignment development and manufacturing (CDMO) from various biotech and global pharmaceutical companies.
This is proof that the company's iPSC technology is recognized not only in Korea but also abroad. Development and production of somatic cells desired by customers from various iPSC cell lines cannot proceed without confidence in iPSC handling technology, so I believe that a lot of experience, knowledge and know-how are required."
It's a new drug development venture company, but why did you design the business model before going public?
"The company fundamentally started as a biotech that applied iPSC-based technology, and was able to naturally expand to new drug development while researching iPSC technology. Considering the time required for new drug development and the size of research funds, we focused on the fact that it is very important for the company to develop its own stamina to support itself along with new drug development. Efforts were made to identify future markets where iPSC technology could generate revenue. In addition, it was decided to strengthen packaging with the goal of diversifying indications for pipeline (new drug candidates) and technology transfer.
The company currently has business models such as △global supply of iPSC-derived somatic cell and organoid products, △toxicity and efficacy screening of new drug candidates and drugs, △iPSC-derived CDMO service, △discovery of new drug candidates linked to iPSC technology. After listing, we aim to become a global leading company in the field of △in vitro screening using organoids, △discover iPSC cell therapy products, and △promote continuous pipeline development."
#2. Established joint venture Celologics...Global Market Entry
It entered the US market by establishing a joint venture (JV) Celologics with US biotech Curibio. What is the difference between a joint venture?
"In October 2021, Nexel established a local joint venture (JV), Celogics, with Seattle-based biotech Curi Bio. Curibio is a company co-founded by Professor Deok-ho Kim of Johns Hopkins School of Medicine, and has a platform (Verification of safety and efficacy of new drugs through the formation of 3-D structures of human iPSC-derived cardiomyocytes and muscle cells) for inducing maturation of iPSC-derived cells.
The needs of Curibio's customers required customization of cardiomyocyte products, such as genetic manipulation, and through discussions with Nexel on new product development, it was determined that the company's sales channels could be rapidly expanded by professionally establishing such a business model. Celologics, a joint venture (JV), was established with Curibio to target the US market.
Celologics is currently focused on selling the company's iPSC-derived cardiomyocytes, and is responsible for sales and support from rebranding to North American and European markets, which has been the foundation for the rapid growth of sales revenue last year and this year. In addition, by securing a site in the Seattle area in the United States, we are aiming to complete the highest level of production and test facilities in 2025. This scalability is the next step of the existing business model, and we will not only build mass production facilities to increase sales volume, but also leap forward as an induced pluripotent stem cell CDMO company at the cGMP (Good Manufacturing Practice) level."
Last March, Nexel completed phase 1 clinical administration of NP-011 for myocardial infarction. What is the plan for the next phase 2 clinical trial?
"Currently, both single administration (SAD) and repeated administration (MAD) of the phase 1 clinical trial of NP-011 for normal people have been completed, and final reports such as clinical statistical analysis are in progress. It is possible to check the topline results in June. Based on the safety results from the phase 1 clinical trial, we are establishing a strategy to enter the phase 2 clinical trial.
However, considering the financial situation and the possibility of realizing future sales targets, we plan to strategically decide when to enter phase 2 clinical trials.Efforts are being made for global technology transfer (L/O) based on efficacy in various indications and phase 1 clinical results. In addition to this, we are reviewing all of the various business models including cooperative projects."
#3. Goal to be listed on KOSDAQ in 2024...Pursuit of 'Make a better place' organizational culture
Please tell us the company's sales target for this year and the schedule for future IPO (Initial Public Offering).
"It was only 600 million won in sales in 2021, but last year, we achieved sales of 1.89 billion won. It achieved sales of 940 million won in the first quarter of this year, about 50% of last year's sales. If this trend continues, I think it will be possible to achieve at least 5 billion in annual sales. The global market is growing as the guideline revision of the ICH (International Committee for Harmonization of Pharmaceutical Regulations) is progressing, which is reflected in the increase in sales.
Last March, we signed a distributor contract with GV Research Platform (GVRP), a non-clinical research service provider in India. In April, we completed a business contract with GOB (Guangdong Organoid Biotechnology) and are preparing to establish a joint venture (JV). After confirming sales performance in the first half of this year, we plan to submit a claim for preliminary examination for listing on the KOSDAQ within the year after undergoing a technical evaluation in the third quarter. We aim to be listed on the KOSDAQ in the first half of 2024."
Please introduce Nexel's unique organizational and research culture.

Researchers at Nexel are constantly striving to introduce new ideas and approaches and to find original solutions that are not bound by conventional methods. / Photo = Nexel
"The company pursues the philosophy of 'Make a better palce', and is committed to creating an organizational culture that values mutual communication while respecting each other's lives. We believe that working together with people from diverse backgrounds and experiences leads to creative and effective results. Through this, we are building a corporate culture that accepts various perspectives and ideas. The company aims for minimal regulation and maximum flexibility. In addition, there is a welfare benefit that provides 3 months paid vacation after 5 years of service.
Regarding research culture, we have a research environment where a rigorous but creative mind coexists from product production to research. Research is conducted with the goal of long-term results rather than short-term results. The company provides a collaborative research environment, helping researchers from various fields of expertise to share ideas and solve problems.
We also provide education and research capacity building programs to acquire the latest research trends and knowledge. We emphasize scientific rigor and transparency in our research activities. To this end, researchers are constantly striving to introduce new ideas and approaches, and to find original solutions that are not bound by conventional methods."

