Nexel Announces Results of Cardiac Safety Pharmacology Research at the World Society of Safety Pharmacology
관리자
view : 702
Nexel, a company specializing in 2D and organoid derived from human induced pluripotent stem cells (hiPSC), announced the results of heart safety pharmacology research using its products at the annual meeting of the Safety Pharmacology Society (SPS) in Brussels, Belgium, on the 20th.
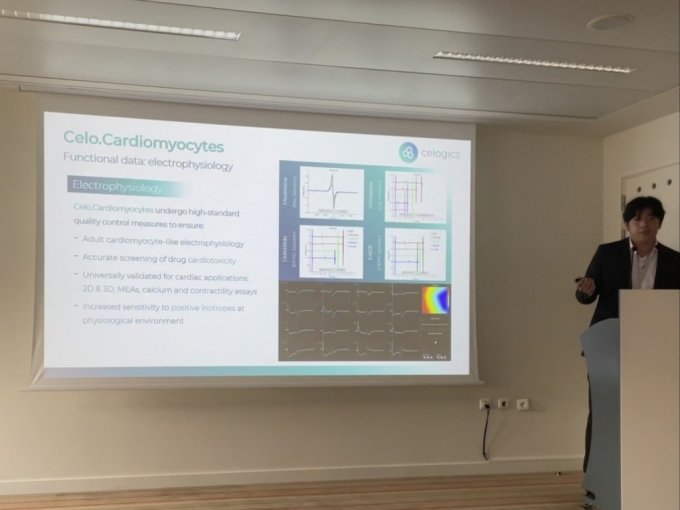
At this conference, Jin-hyuk Chang, CEO of Celogics, presented under the theme of "Enhancing hiPSC-CM Electrophysiology/CiPA 28 with Serum-Protein Free Media."
In particular, it succeeded in developing Serum-Protein-Free media, which is having difficulty developing at its competitor FujiFilm CDI, and drew attention from the audience by announcing that it drew outstanding evaluation results from various heart safety measuring equipment.
"Serum-Protein-Free media does not use animal serum and cytokine for research using human heart cells," a company official said. "It fits not only the economic feasibility and accuracy of research, but also the atmosphere of society to reduce the use of animal-derived substances in research."
In addition, the company explained that in the evaluation conducted by the researchers of Professor Godfrey Smith of the University of Glasgow, who is an expert in cardiac safety pharmacology, Nexel's cardiomyocytes cells showed good Signal to Noise Ratio (SNR) to competitive products, and a slight change in activity potential due to drug treatment was also detected in detail, resulting in a highly reliable drug evaluation.
The official stressed, "It is meaningful that we have obtained high reliability results from a third institution even in areas that cannot be analyzed with existing competitors' myocardial cell products."
Meanwhile, Celogics is a joint venture co-founded with Curi Bio, a U.S. biotech company, in 2021. NEXEL's hiPSC-derived somatic cell products are sold in the United States and Europe and commissioned development and production (CDMO) of hiPSC cells.
Nexel is a biotech that produces hiPSC-derived somatic cell products, conducts new drug screening services using cardiac safety pharmacology and organoids, commissioned development production (CDMO), and develops new drugs for hiPSC-derived proteins and peptides. As part of the globalization of sales, it is committed to entering the US, India, Japan, China, and UK markets.

